1) List the known and unknown quantities.
Sample: luminol.
Mass: 10.0 g.
Volume: 75.0 mL.
Molarity: unknown.
2) Set the equation.
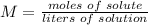
3) Conversion of units.
3.1-Convert mass to moles.
The molar mass of luminol (C8H7N3O2) is 177.1601 g/mol.
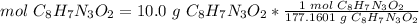
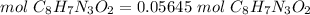
3.2-Convert mL to L.
1 L = 1000 mL
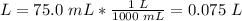
4) Plug in the known quantities.
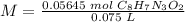

The molarity of the stock solution is 0.75 M C8H7N3O2.
.