Answer:
To determine the heat needed to melt 40g of ice we use the following formula:
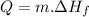
Where:
m is the mass of water
Hf is the heat of fusion
In this case we consider that the process hapens at 0°C.
The average heat of fusion is:
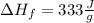
So we calculate:
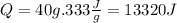
Now we convert the value to kJ:
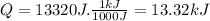
So the answer is 13.32kJ.