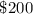Answer:
Step-by-step explanation:
From the graph in the attached image of the question.
We can see that the starting value of the amount in account at month zero is;
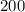
Therefore, at the start of the year, Alex already have $200 in his account.
So, at the start of the year the amount of money in Alex's account is;