To find the hydrogen ions concentrations, we use the following formula.
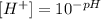
(a) When pH = 2.42, we obtain the following.
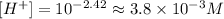
(b) When pH = 11.21.
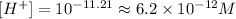
(c) When pH = 6.96.
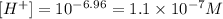
(d) When pH = 15.00.
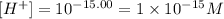
Therefore, the hydrogen ion concentration for each case is
• 3.8x10^-3 M.
,
• 6.2x10^-12 M.
,
• 1.1x10^-7 M.
,
• 1x10^-15 M.