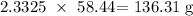Answer:
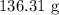
Step-by-step explanation:
Here, we want to get the mass required to make the given volume
To get this we need to get the number of moles
To get the number of moles, we have to multiply the volume by the molarity
Mathematically, we have that as:
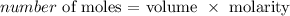
The volume can be converted to liters by dividing by 1000
So we have the volume as 311/1000 = 0.311 L
Thus, we have the number of moles as:
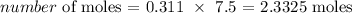
To get the mass, we have to multiply the number of moles by the molar mass
The molar mass of NaCl is 58.44 g/mol
Thus, we have the mass as: