Step-by-step explanation:
The equation t(c) = -3,970.9 (ln c) relates the age of a plant fossil t and the percentage of carbon-14 c.
So, To know the age of a plant fossil with 47% of its carbon-14 remaining, we need to replace c by 0.47 and calculated t(c) as:
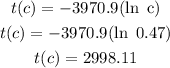
Therefore, A fossil with 47% of its carbon-14 remaining is approximately 2998 years old.
On the other hand, to know the percentage of carbon remaining in a fossil that is 7,000 years old, we need to replace t(c) by 7,000 and calculate the value of c. Then:
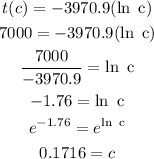
Therefore, A fossil that is 7,000 years old will have approximately of its carbon-14 remaining.