The question requires us to balance the equation for the reaction between Na3PO4 and KOH.
To balance a chemical reaction, we need to find coefficients to achieve the same number of atoms of each element on both sides of the equation.
A simple way to balance an equation is follow the order metals > nonmetals > carbon > hydrogen > oxygen.
Given the information above, we'll proceed to balance the equation given.
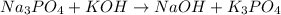
There are 3 atoms of sodium (Na) on the left side of the equation, thus we can change the coefficient for NaOH from 1 to 3 to have the same amount of Na atoms on the right side:
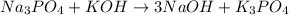
To balance the amount of potassium (K), another metal, we can change the coefficient of KOH from 1 to 3 to achieve three atoms of K on both sides of the equation:
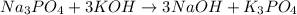
Now, let's check the amount of atoms before moving on to balancing hydrogen and oxygen:
Na: 3 atoms on the left side and 3 atoms of the right side
K: 3 atoms on the left side and 3 atoms on the right side
P: 1 atom on the left side and 1 atom on the right side
H: 3 atoms on the left side and 3 atoms on the right side
O: 7 atoms on the left side and 7 atoms on the left side
Therefore, the equation is balanced just by changing the coefficients for KOH and NaOH.
The following coefficients are required to answer the question: 1, 3, 3, 1