Answer:
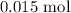
Step-by-step explanation:
Here, we want to get the number of moles of NO gas that can be formed
To answer this, we need to be sure of the limiting reagent
The limiting reagent is the one that is responsible for the number of moles of product formed
From the shown balanced equation of reaction, 3 moles of silver produced 1 mole of the gas
Thus, 0.1 mol of powdered silver will produce:
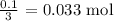
For the nitric acid, we need to get the number of moles that reacted
To get this, we have to multiply the volume (in L) that reacted by the molarity
Mathematically, we have that as:
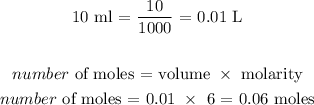
From the equation of reaction, 4 moles of the nitrate gave 1 mole of the gas
Thus, we have it that 0.06 moles will give:
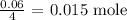
From what we see, the number of moles produced by the nitrate is less than what was produced by the solid
Thus, the nitrate is the limiting reagent and the number of moles of the gas produced is 0.015 mol