For this exercise we assume ideal gas to use the ideal gas law:
p x V = n x R x T (1)
From this equation, we clear T, where:
p = pressure in atm
V = volume in L
n = moles
T = temperature in K
R = gas constant (we can find this on books, the Internet, and even students can ask the teacher about its value)
R in this case and because of units is equal to 0.082 atm x L / mol x K
Procedure:
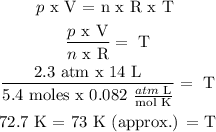
Answer: T = 73 K