Answer:
![\begin{gathered} a)\text{ \lbrack H}^+]\text{ = 0.0112 mol/L} \\ b)\text{ pH = 1.95} \\ c)\text{ pOH = 12.05} \\ d)\text{ \lbrack OH}^-]\text{ = 8.91 }*\text{ 10}^(-13)\text{ mol/L} \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/km59ogp19n6hogtrchg3o6gjhbzhh144ge.png)
Step-by-step explanation:
We start by getting the hydrogen ion concentration:
a) [H+]
Since formic acid is not a strong acid, it does not ionize completely into hydrogen ion
We have to use its Ka and an ICE table to calculate the number of moles of the oxonium ion
We proceed as follows:
The equation of reaction is:
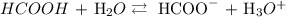
We have the ICE table as follows:
I is the initial stage
C is the change in concentration
E is the equilibrium state
At the equilibrium state, we have the equilibrium constant of the acidic dissociation as follows:
![K_a\text{ = }([H_3O^+][HCOO^-])/([HCOOH])](https://img.qammunity.org/2023/formulas/chemistry/college/tuwkxeb02cssu1bwcixmjvtwc12nnyf7y5.png)
We substitute the equilibrium concentration and the Ka value given
We have that as:
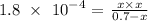
At equilibrium, the value of x will be very small
Thus:
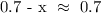
Thus:
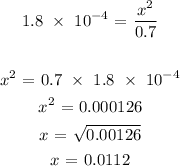
Thus, the concentration of the hydrogen ion is 0.0112 mol/L
b) We can get the pH from here
![\begin{gathered} pH\text{ = -log\lbrack H}_3O^+] \\ pH\text{ = -log\lparen0.0112\rparen} \\ pH\text{ = 1.95} \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/vtlgpyol522pcvg24lcgrvgvkwvds74fol.png)
c) Let us get pOH
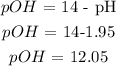
d) Lastly, we want to get the concentration of the hydroxide ion
Mathematically:
![\begin{gathered} pOH\text{ = -log\lbrack OH}^-] \\ [OH^-]\text{ = 10\textasciicircum\lparen-pOH\rparen} \\ =\text{ 10\textasciicircum\lparen-12.05\rparen} \\ =\text{ 8.91 }*\text{ 10}^(-13) \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/bnsd26k4ygx2ezne56y57jpoowdr3uzg0n.png)