ANSWER
False
Step-by-step explanation
Firstly, write the ionic equation of NaCl
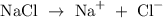
The atomic number of sodium atom is 11 and the atomic number of chlorine is 17
Recall, that sodium is a metal and chlorine is a non metal
The electronic configuration of sodium and chlorine atom is given below as
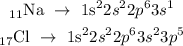
In the above configuration, sodium has one electron at it outermost shell and chlorine has 7 electrons at it outermost shell.
Hence, sodium is attracted to 7 chloride ions for the both elements to attain it structure.
Therefore, sodium ion is attracted to 7 chloride ion.
The statement is false