Step 1
Latent heat is used to change a phase of a substance to another one.
Mathematically:
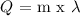
Where,
Q = heat
m = mass of substance
λ = the heat of vaporization
-------------
Step 2
Information provided:
Q = 59.5 J (a positive value because heat is absorbed)
m = 102 g
λ = unknown
Procedure:
Q = m x λ
Q/m = λ
59.5 J/102 g = 0.58 J/g
Answer: λ = 0.58 J/g