Answer
Mass of water (m) = 14.76 g
Step-by-step explanation
Given:
Balanced chemical equation
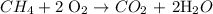
Volume of CH4 = 10 L
Required: Mass of water produced
Solution:
Step 1: Find the mass of CH4
density = m/V
m = density x V
m = 0.657 g/L x 10 L
m = 6.57 g
Step 2: Find the moles of CH4
n = m/M n is the moles, m is the mass and M is the molar mass
n = 6.57g/16,04 g/mol
n = 0.409 mol
Step 3: Use stoichiometry to find the moles of H2O
The molar ratio between CH4 and H2O is 1:2
Therefore the moles of H2O = 0.409 x 2 = 0.819 mol
Step 4: Calculate the mass of H2O
n = m/M
m = n x M
m = 0.819 mol x 18,015 g/mol
m = 14.76 g