Answer:
The percentage of of abundance of the isotope 34.97 amu is 76.00%.
Step-by-step explanation:
To calculate the percentage of abundance of the isotope 34.97 amu, it is necessary to write an equation, assuming that the isotope with mass 34.97 is called Cl-35 and that isotope with mass 36.97 is called Cl-37. We can represent them like:
- Cl-35: x
- Cl-37: 1-x
Each isotope multiplied by its percentage and added will be equal to the average atomic weight of chlorine (35.452), so the equation is:
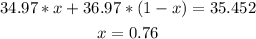
After solving the equation, we find that x=0.76, so the percentage of Cl-35 is 76.00% and the percentage of Cl-37 is 24.00% (1-0.76=0.24).