There are 2.71*10^24 atoms of oxygen.
1st) It is necessary to calculate the amount of oxygen in 1.50mol of sodium carbonate (Na2CO3).
We know that 1 mole of sodium carbonate has 3 moles of oxygen, so to calculate the moles of Oxygen in 1.50 moles of Na2CO3 we can use a mathematical Rule of Three:
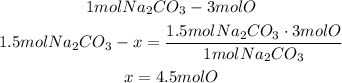
Now we know that there are 4.5 moles of oxygen in 1.50 moles of sodium carbonate.
2nd) Finally, we can calculate the atoms of oxygen using the Avogadro's number (6.022*10^23 atoms/mol):
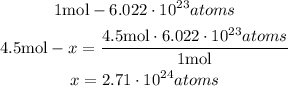
So, there are 2.71*10^24 atoms of oxygen in 1.50 mol of sodium carbonate.