Answer: 9.00g of H2O are produced in the reaction when 31.8g of Cu are completely consumed
Step-by-step explanation:
The question requires us to calculate the mass of H2O obtained when 31.8g of Cu are completely consumed, according to the chemical reaction between Cu and HNO3:
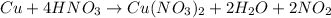
To solve this problem, which is a mass to mass stoichiometry problem, we'll need to follow the steps:
mass of Cu → moles of Cu → moles of H2O → mass of H2O
Note that the chemical equation provided is already balanced, thus we can use it as it was given.
First, let's calculate the number of moles of Cu that corresponds to 31.8g of this metal, knowing that the molar mass of metallic copper is 63.546 g/mol:
63.546g Cu ------------------ 1 mol Cu
31.8g Cu ---------------------- x
Solving for x, we'll have:
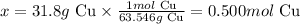
Therefore, we can say that 0.500 moles of Cu were used in the reaction.
Next, let's calculate the numer of moles of H2O that would be produced, considering the amount of Cu used and the stoichiometry of the reaction.
According to the balanced chemical equation, 1 mol of Cu is necessary to produce 1 mol of H2O:
1 mol Cu ---------------- 1 mol H2O
0.500 mol Cu -------- y
Solving for y, we'll have:
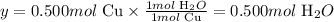
Therefore, we can say that 0.500 moles of H2O were produced in the reaction.
At last, let's calculate the mass of H2O that corresponds to 0.500 moles of it, knowing that the molar mass of H2O is 18.004 g/mol:
1 mol H2O ------------------ 18.004g H2O
0.500 mol H2O ---------- z
Solving for z, we'll have:
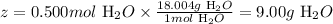
Therefore, we can say that 9.00g of H2O are produced in the reaction when 31.8g of Cu are completely consumed.