Answer
24.325 mol
Step-by-step explanation
The unbalanced equation is:
C₂H₆ + O₂ → CO₂ + H₂O
The balanced equation will be:
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
Given mole of ethane = 6.95 mol
From the balanced equation:
2 mol of C₂H₆ is required by 7 mol of O₂
Therefore, 6.95 mol of C₂H₆wills requir:
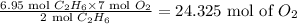
Hence, 24.325 mol of O2 is required.