Answer:
1. combustion
2. decomposition
3. decomposition
4. combination
5. decomposition
6. decomposition
Step-by-step explanation:
combination of a reaction is when 2 more or elements/ compounds combine to form a compound. example: (A+B→AB)
decomposition of a reaction is when a chemical breakdown. example: (AB→A+B)
a combustion reaction is when an element/ compound reacts with oxygen to form the product of water and carbon dioxide.
example: (
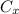
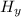 +
+
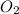 →
→
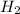 O +
O +
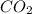 )
)