29. We must bear in mind that the absorbance is directly proportional to the concentration. If the absorbance is reduced by half this means that the concentration presented a reduction of 50% as well. Now, we have an initial absorbance, t=0. This will be when the reactant, C25H30N3, concentration is 100%.
The absorbance value for a 100% initial amount is 0.62, with a rule of three we can find the absorbance that we would have if 75% reacts.
The absorbance shows us the amount of C25H30N3 that remains, that is, the amount that does not react. This value will be equal to:
100%-75%=25%
Now, with a rule of three, we calculate the absorbance that the solution should have by 25% of C25H30N3 compared to the initial concentration.
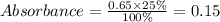
Now, if we see the data obtained, the time in which this absorbance is obtained is 300 seconds.
So, for question 29 the answer will be: (C) 300 s
30. Now, we are told that the first-order reaction with respect to C25H30N3+ is first order. This means that the rate of the reaction is directly proportional to the concentration. If the C25H30N3+ solution is diluted, the concentration will also decrease. Therefore, the reaction rate will be lower.
For question 30 the answer will be: (B) It will be lower