Step-by-step explanation:
Stoichiometry is the quantitative study of the mass relation of reactants and products in a chemical reaction to determine the amount of reactant required for a reaction to produce a specified quantity or to determine the theoretical yield for the products.
The mole is an SI base unit to measure the amount of elementary entities (atom, molecules, ions, electrons, particles, etc.) of a given mass of a particular substance. One mole is the quantity of a substance exactly equal to Avogadro's number which is approximately
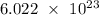 elementary entities.
elementary entities.
Using dimensional analysis, we can evaluate the mass of nickel (II) carbonate as shown below.
![\text{mass}_{\ \text{NiCO}_(3)} \ = \ 7.46 \ * \ 10^(23) \ \text{formula units} \ * \ \displaystyle\frac{1 \ \text{mol}}{6.022 \ * \ 10^(23) \ \text{formula units}} \\ \\ \-\hspace{2.5cm} * \display\style\frac{\left[58.69 \ + \ 12.01 \ + \ 3(16.00) \ \right] \ \text{g}}{1 \ \text{mol}} \\ \\ \-\hspace{1.85cm} = 147 \ \text{g} \ \ \ \ \ \ \ \ (3 \ \text{s.f.})](https://img.qammunity.org/2023/formulas/chemistry/college/ydx1v3zubr30sh9vi2nlgc919h55de79g3.png)