The solution of 0.026 K2SO4 has the greatest concentration of K+.
Through the dissociation equation of each compound we can see that:
0.013M K3PO4
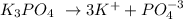
0.013M 3x(0.013)
So, the K+ concentration is 0.039M.
0.026M K2SO4
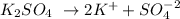
0.026 2x(0.026)
Here the K+ concentration is 0.052M.
0.032M KNO3
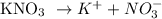
0.032 0.032
The concentration of K+ is 0.032M.
0.014M KBr
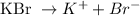
0.014 0.014
Finally, here the K+ concentration is 0.014M.