Answer: the pOH of this solution is 9.7
Step-by-step explanation:
The question requires us to calculate the pOH of a solution, knowing that the pH of this same solution is 4.3.
Due to the ionic product of water (Kw = 1.00 x 10^-14 at 25°C and Kw = [OH] x [H+]), we can say that,
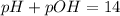
Thus, applying the value of pH given by the question to the equation above, we'll have:
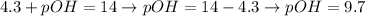
Therefore, the pOH of this solution is 9.7.