To solve this problem we are going to assume that the temperature at which the gold is found is room temperature.
We are going to use a property of materials that relates mass to volume, this is density. At this temperature, the density of gold is 19.3g/mL, the equation of density is:
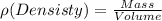
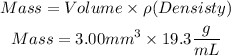
We will use the following conversion factors:
1mL=1000mm^3
1atom=3.271x10^-22g
So, the atoms present in 3.00mm^2 will be:
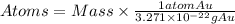
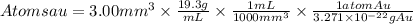
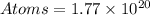
In 3.00mm^3 of gold, there are 1.77x10^20 atoms
Answer: 1.77x10^20 atoms