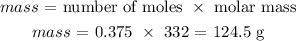Answer:
Step-by-step explanation:
Here, we want to get the mass of silver chromate produced
Since silver nitrate is in excess, it means that Silver chromate is the limiting reactant
Now, we need to get the number of moles of silver chromate that reacted
Mathematically:
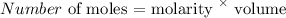
From the question:
molarity is 1.5 M
volume is 250 mL (250/1000 = 0.25 L)
Thus, we have the number of moles as:
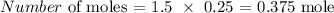
Looking at the equation of reaction, 1 mole of sodium chromate produced 1 mole of silver chromate
Thus., 0.375 mol of sodium chromate will produce 0.375 mol of silver chromate
Now, to get the mass of silver chromate produced, we have to multiply the number of moles by the molar mass of silver chromate
The molar mass of silver chromate is 332 g/mol
Mathematically: