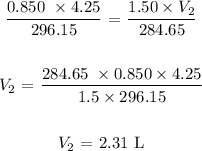Answer:
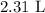
Step-by-step explanation:
Here, we want to calculate the final volume
We use the general gas equation here:
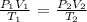
P1 is the initial pressure which is 0.850 atm
V1 is the initial volume which is 4.25 L
T1 is the initial temperature which is (23 + 273.15 = 296.15 K)
P2 is the final pressure which is 1.50 atm
V2 is the final volume which is unknown
T2 is the final temperature (11.5 + 273.15 = 284.65 K)
Substituting the values, we have: