The formula to find the specific heat capacity is
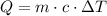
Where Q is the heat, c is the specific heat capacity, m is the mass, and T represents the variation of the temperature. Use the given magnitudes to find c.
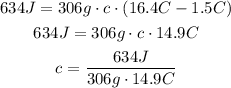
But, we need to express 14.9°C in Kelvin, just add 273.15.
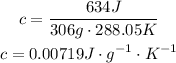
Therefore, the chemist can report a heat capacity of 0.00719.