Answer
19.93 moles
Step-by-step explanation
Given:
The number of particles of MgO = 1.2 x 10²⁵ particles
What to find:
The number of moles present in 1.2 x 10²⁵ particles of MgO.
Step-by-step solution:
Let the number of moles of MgO present be x.
From Avogadro, 1 mole of any substance contains 6.022 x 10²³ particles
This means 1 mole of MgO contains 6.022 x 10²³ particles,
So, x mole of MgO will contain 1.2 x 10²⁵ particles of MgO
To get x, cross multiply and divide both sides by 6.022 x 10²³ particles as shown below:
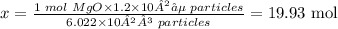
Therefore, the number of moles present in 1.2 x 10²⁵ particles of MgO is 19.93 moles