Answer: the reaction given by the question can be classified as a neutralization reaction.
Step-by-step explanation:
The question requires us to classify the following chemical reaction:
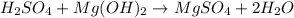
The reaction between sulfuric acid (H2SO4) and magnesium hydroxide (Mg(OH)2) to form a salt, magnesium sulfate (MgSO4) and water (H2O) can be compared to the following generic neutralization chemical equation:
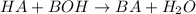
where the acid HA reacts with the base BOH to form a salt, BA, and water.
Also, note that H2SO4 and Mg(OH)2 can be classified as an acid and a base, respectively.
Therefore, the reaction given by the question can be classified as a neutralization reaction.