Given,
The initial pressure of the gas, P₁=120000 N/m²
The initial temperature of the gas, T₁=300 K
The required pressure of the gas, P₂=180000 N/m²
From Gay-Lussac's law,
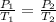
Where T₂ is the required teperature to achieve the given gas pressure.
On substituting the known values,
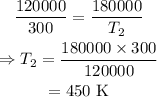
Thus the alarm is activated at the temperature of 450 K
(i)
When the temperature of the gas is increased the kinetic energy of the molecules of the gas increases. This results in increased collision between the molecules. Increased collision causes the pressure of the gas to increase. Due to this pressure, the piston is pushed and the alarm goes off.