Given:
Volume of water = 2.5 L
Initial temperature, T1 = 0°C
Final temperature, T2 = 87.0°C
Let's find the amount of heat needed in Joules.
Apply the specific heat Capacity formula:
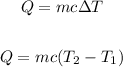
Where:
C is the specific heat capacity of water = 4.187 kJ/g °C
To find the mass, m, we have:
1 L = 1 kg
2.5 L = 2.5 kg
Therefore, we have:
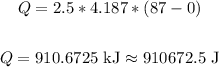
Therefore, the amount of heat needed is 910672.5 Joules.
ANSWER:
910672.5 J