To solve the question we will use Charles's law, which relates volume and temperature when pressure is kept constant.
Charles's law says the following:
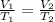
Where,
V1 is the initial volume = 0.128L
T1 is the initial volume = 240K
T2 is the final volume = 198K
V2 is the final volume = ?
Now, we clear V2 and replace the known data:
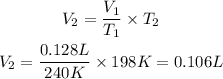
Answer: At 198K if the pressure remains constant the volume of the gas will be 0.106L