To answer this question, we have to use the heat of vaporization of water, that tells us the amount of energy required to evaporate or condense 1 g of water.
According to it, you need to remove 2.257kJ to condensate 1 g of water:
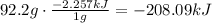
It means that -208.09kJ are required to condensate 92.2grams of water.
The answer is negative because to condensate a substance you have to remove heat, so when we express it we have to write as a negative amount.