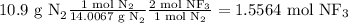Considering the following equation and that Fluorine is in excess to allow all the molecular nitrogen to react
N₂ + 3F₂ → 2NF₃
We will use the molar mass and reaction stoichiometry to determine the grams of NF₃ produced, using the following calculation: