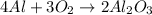To balance an equation, we have to add coefficients to the reactnts and products so that we have the same number of each element on both sides.
For now, we have 1 Al on the left side and 2 on the right side.
Also, we have 2 O on the left side and 3 on the right side.
Since we have 1 Al alone on the left side, it is easy to balance it, so we can left for last.
So, lets balance O first. Since we have 2 on the left side and 3 on the right side, we can add coefficients switched: 3 on O₂ and 2 on Al₂O₃. Tha way, we wil have 6 on both sides:
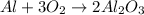
Now, we have to balance Al. We have 1 on the left side still and now we have 4 on the right side. So, we can add a coefficient of 4 on Al Since this don't change the number of O on either side, this won't affect the previous balancing.
So, the balanced equation is: