The question requires us to calculate the volume of oxygen in a cyllinder of this gas, given the initial volume and pressure and final pressure of the tank and considering the system under constant temperature.
The following information was provided by the question:
Initial volume of gas = 5.0 L
Initial pressure = 9.0 atm
Final pressure = 1.0 atm
To solve this question, we can consider the combined gas law, which, for a susbtance under two different sets of conditions, can be written as:
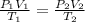
where P is the pressure, V is the volume, T is the temperature (in Kelvin) and 1 and 2 refers to the intial and final conditions, respectively.
Since the temperature is maintained constant in the system given by the problem, we can use the gas law as:
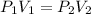
Furthermore, as the question requires us to calculate the final volume of the gas, we can rearrange the equation as:
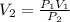
Now, considering the information provided by the question, we can calculate the final volume of oxygen in the tank:
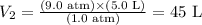
Therefore, under the given conditions, the final volume of oxygen would be 45 L.