To answer this question, we have to use the Charles' Law of gases, that states the following:
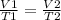
In this case, we know the values of T1, V1 and T2 and we have to find V2:
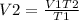
Replace for the given values and find V2:
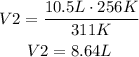
It means that the volume of the balloon outside is 8.64L.