Answer: An element with charge +3, 10 core electrons and 5 valence electrons would represent an element with atomic number 15 (10 core electrons + 5 valence electrons). According to the periodic table, this element would be Phosphorus (P).
Step-by-step explanation:
The question requires us to identify an element that presents charge +3, 10 core electrons and 5 valence electrons.
First, let's determine the electron configuration for an atom with 10 core and 5 valence electrons.
Considering the build-up principle and the Pauling's diagram, we can write the electronic configuration as:
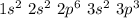
Note that the electrons 1s2, 2s2 and 2p6 correspond to the core electrons, while 3s2 and 3p3 are the valence electrons.
Next, we need to consider the cation that would be formed by this atom, when it "loses" 3 electrons:
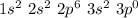
Therefore, an element that forms a cation with charge +3 and contains 10 core electrons and 5 valence electrons would represent an element with atomic number 15 (10 core electrons + 5 valence electrons). According to the periodic table, this element would be Phosphorus (P).