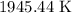Given:
The molecular mass of the molecule is
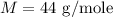
The speed of the molecule is
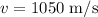
Required: The temperature of the molecule is
Step-by-step explanation:
we know that root mean square velocity of the molecule is given as
![v=\sqrt[2]{(3kN_AT)/(M)}](https://img.qammunity.org/2023/formulas/physics/college/oc9a7l4h7y0ud0lbyhbogq24tcj7snjzib.png)
Here, k is Boltzmann's constant and m is the mass of the molecule in grams and T is the temperature and
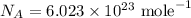
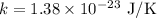
we have to find T then square the above relation and simply for T
![\begin{gathered} v=\sqrt[2]{(3kN_AT)/(M)} \\ v^2=(3kN_AT)/(M) \\ T=(v^2M)/(3kN_A) \end{gathered}](https://img.qammunity.org/2023/formulas/physics/college/wq4v6qe61n6z33khrl9mxc1mnph7y34s3w.png)
now change the molecular mass into standard units

now plugging all the values in the above relation we get

Thus, the Temperature of the molecule is