The exponential decay is given by:
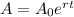
where A0 is the initial amount of the element and r is the decay rate.
To find the decay rate we use the fact that the half life is 1590 years; this means that it takes 1590 years for the amount of substance to be half the original amount, that is:
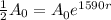
Solving for r we have:
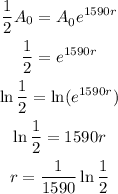
Hence the decay rate is:
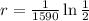
Now that we have the decay rate we have that the function describing the amount of radium for our example is:
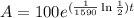
To determine how much radium we have after 1000 years we plug t=1000 in the function above:
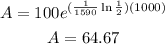
Therefore after 1000 years we have 64.67 mg of radium-226