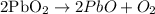To balance this equation, we have to write out the chemical formula of lead(iv)oxide.
The chemical formula of lead(iv)oxide is
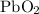
The decomposition of lead(iv) oxide will occur when heated at a high temperature to produce lead(ii) oxide.
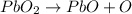
Let us balance the reaction above.
Applying the law of conservation of matter i.e
Since we have 1 atom of Pb in the reactant side and 1 atom of Pb in the product side, that should be balanced.
But the oxygen isn't yet balanced.
Add 2 in front of both sides i.e PbO₂ and PbO to make the equation balanced.
The equation would become
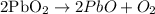
If we look at the Pb
reactant = 2
product = 2
for the oxygen side
reactant = 4
product = 4
The balanced equation to represent the decomposition of lead(iv)oxide is given as