Answer
(a) 10(±0.1 °C)
Step-by-step explanation
(a) Temp. change of metal (±0.1 °C)
To find the temp. change of metal (±0.1 °C), you need to first determine the averages of the initial and final temperatures of the water:
The average initial temperature, T₁ =
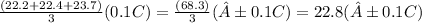
The average final temperature, T₂ =
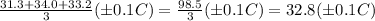
Therefore, the temp. change of metal (±0.1 °C) ΔT = T₂ - T₁
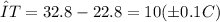
(b) Heat absorbed by water (Joule).
The formula to calculate the heat absorbed by water in joule is:
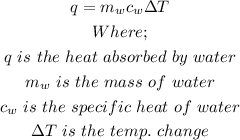
The average mass of water is
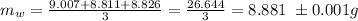
The specific heat of water is