Answer:
![sulfur\text{ d}\imaginaryI\text{ox}\imaginaryI\text{de + water}\operatorname{\rightarrow}sulfurous\text{ ac}\imaginaryI\text{d.}]()
Step-by-step explanation:
We hav an acid oxideareacting with water. Why do we call sulfr dioxide as oacid oxide=? Because acid oxides are formed by a nonmetal (sulfr) with oxygen .
When we react an acid oxide with water, we will create an acid that contains hydrogen (H), the nonmetal (in this case, S), and o.ygen
Remember that sulfur dioxide is SO2 and water is H2O. So the chemical equation will be:
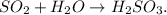
And you can realize that the cemical equation is already balanced,. H2SO3 is called sulfurous acid because of the oxidation state that we're using of S, which is +4. Remember that if we use +6 as oxidation state, we will call sulfuric acid and if we use +2 as oxidation state, we will call hyposulfurous acid,so the answer is:
![\begin{gathered} SO_2+H_2O\operatorname{\rightarrow}H_2SO_3, \\ sulfur\text{ dioxide+water}\rightarrow sulfurous\text{ acid.} \end{gathered}]()