Answer:
2.0atm
Explanations:
According to Henry's law, the solubility of a gas in a liquid is directly proportional to its partial pressure of the gas above the liquid. Mathematically;
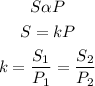
Given the following parameters
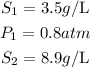
Substitute the given parameters into the formula to have:
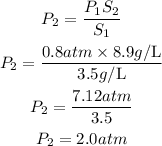
Hence the pressure needed to produce an aqueous solution containing 8.9 g/L of the same gas at 0 degrees Celsius is 2.0atm