Step 1 - Finding the relation in moles in the reaction equation
As we have previously seen working on a similar problem, the bigger numbers that come before the formula of the substance expresses a proportion in moles between all the reactants and the products.
The reaction given in this exercise is:
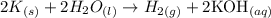
Note that 2 moles of K react with 2 moles of H2O, producing 1 mole of H2 and 2 moles of KOH.
Step 2 - Converting a relation in moles to a relation in grams
We can convert this relation in moles to a relation in grams by multiplying each number of moles by the molar mass of each substance. The molar masses are:
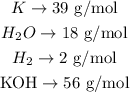
Converting to grams:
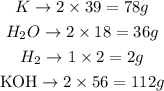
We can state thus that 78g of K react with 36g of water, producing 2g of H2 and 112g of KOH. This is a fixed proportion, like a cake recipe.
Step 3 - Using the information to answer the exercise
1) Note that 2 moles of K produces 2 moles of KOH. Therefore, we would need exactly 2 moles of K.
2) Note that 2 moles of K, as we have previously calculated in step 2, corresponds to 78 g.
3) Note that 2 moles of K produces 1 mole of H2. Since this is a fixed proportion, we can set the following relation:
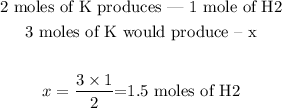
It would be produced thus 1.5 moles of H2.
4) As we have seen in 3), we would produce 1.5 moles of H2. Since its molar mass is 2g/mol, we would produce, in grams:
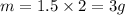
3g of H2 would be produced.
5) Note that 2 moles of H2O produce 1 mole of H2. In grams, this corresponds to 36g of water produce 2g of H2.