Answer
mass of CO2 required = 60.5 g
Step-by-step explanation
Given:
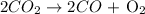
Molar mass of CO2 = 44 g/mol
Molar mass of O2 = 32 g/mol
Mass of O2 = 22 g
Required: Mass of CO2 needed to produce O2
Solution:
Step 1: Calculate the moles of O2
n = m/M where n is the moles, m is the mass and M is the molar mass
n = 22g/32g/mol
n = 0.6875 mol
Step 2: Find the moles of CO2 using stoichiometry
Molar ratio between CO2 and O2 is 2:1
Therefore moles of CO2 = 0.6875 mol x 2 = 1.375 mol
Step 3: Covert the moles of CO2 to mass
m = n x M
m = 1.375 mol x 44 g/mol
m = 60.5 g