Step 1 - Understanding stoichiometry
What we call stoichiometry in Chemistry is nothing but the proportion between reactants and products. This proportion can only be properly obtained when the reaction is balanced, as it naturally arises from the conservation of mass.
Therefore, when we are looking for the stoichiometry of the reaction, we have to look at those big numbers that come before the formula of each substance. Let's see an example:
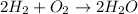
In the reaction above, the stoichiometry, i.e., the proportion between reactants and products is 2 H2 : 1 O2 : 2 H2O, or simply 2:1:2.
Therefore, the information that describes the stoichiometry is always the balanced equation.
Step 2 - Understanding reaction scale
When we refer to reaction scale we mean how much reactant we have used. We can do a small scale reaction, or we can do great scale reactions (an explosion, for example).
The more reactant we use, the greater the scale of the reaction. Therefore, the information that correctly describes the scale of the reaction is the number of moles used as reactants in the experiment.