We will have the following:
We are given:
*Mass empty graduated cylinder: 50g
*Mass of the cylinder has a mass of 120g when Volume of water = 30mL.
*Volume to 75 mL when rock is added and the mass is 250g.
First, we will have that the mass of the rock wilk be give by:
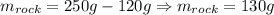
Now, we determine the volume it occupies:
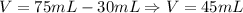
Now, we determine the density of the rock as follows:
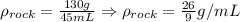
So, the density of the rock is exactly 26/9 g/mL.