Answer:
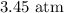
Step-by-step explanation:
Here, we want to get the total pressure of the mixture of gases
Mathematically, we know from the Ideal gas equation, that:
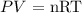
where P is the pressure that we want to calculate
V is the volume of the container which is 55 L
n is the total number of moles of gases in the container that can be obtained by adding the number of moles of oxygen to that of nitrogen. We have the total number of moles as (3 + 4.5 = 7.5 moles)
R is the molar gas constant which is 0.0821 L atm/mol.k
T is the temperature that we convert to Kelvin by adding 273.15 (35 + 273.15 = 308.15 K)
Now, we proceed to substitute these values after re-writing the equation in terms of P
Mathematically, we have that as:
