Answer:
1.33L
Explanations:
According to Charle's law, the volume of a gas is directly proportional to temperature provided the pressure remains the same. Mathematically;
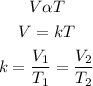
Given the following parameters
Final volume V2 = 2.80L
Initial temperature T1 = 257.9K
Final temperature = 544.9K
Required
Initial volume V1
Substitute the given parameters
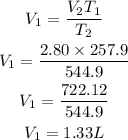
Hence the initial volume in litres is 1.33L