Let's see that the reaction between an acid (H2SO4) and a base (NaOH) forms a salt (Na2SO4) and water (H2O).
You can realize that in the reactants we have 1 mol of Na (sodium) but in the products, we just have 2 moles of Na, so putting '2' moles besides NaOH to balance this element, our equation would be:
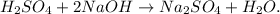
But, hydrogen and oxygen are unbalanced, because in the reactants we have 4 hydrogens and 6 oxygens but in the products, we have 2 hydrogens and 5 oxygens, so we have to put besides H2O a '2' moles, we're going to obtain the balanced equation:
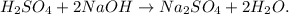
Now, we can see the molar ratio of NaOH and H2O. We have 2 moles of NaOH to 2 moles of H2O, the mole ratio between them is 2:2 or 1:1. This molar ratio can be multiplied by any number for both sides, as you can see in the answer choices, we have the option that 4 moles of NaOH to 4 moles of H2O would be the correct answer because we're multiplying 2 to 2:2 or 4 to 1:1.